Vegetable Disease Diagnostics Report
go.ncsu.edu/readext?244837
en Español / em Português
El inglés es el idioma de control de esta página. En la medida en que haya algún conflicto entre la traducción al inglés y la traducción, el inglés prevalece.
Al hacer clic en el enlace de traducción se activa un servicio de traducción gratuito para convertir la página al español. Al igual que con cualquier traducción por Internet, la conversión no es sensible al contexto y puede que no traduzca el texto en su significado original. NC State Extension no garantiza la exactitud del texto traducido. Por favor, tenga en cuenta que algunas aplicaciones y/o servicios pueden no funcionar como se espera cuando se traducen.
Português
Inglês é o idioma de controle desta página. Na medida que haja algum conflito entre o texto original em Inglês e a tradução, o Inglês prevalece.
Ao clicar no link de tradução, um serviço gratuito de tradução será ativado para converter a página para o Português. Como em qualquer tradução pela internet, a conversão não é sensivel ao contexto e pode não ocorrer a tradução para o significado orginal. O serviço de Extensão da Carolina do Norte (NC State Extension) não garante a exatidão do texto traduzido. Por favor, observe que algumas funções ou serviços podem não funcionar como esperado após a tradução.
English
English is the controlling language of this page. To the extent there is any conflict between the English text and the translation, English controls.
Clicking on the translation link activates a free translation service to convert the page to Spanish. As with any Internet translation, the conversion is not context-sensitive and may not translate the text to its original meaning. NC State Extension does not guarantee the accuracy of the translated text. Please note that some applications and/or services may not function as expected when translated.
Collapse ▲The Vegetable Pathology lab at NC State has been working in collaboration with the Plant Disease and Insect Clinic to diagnose vegetable diseases. We have compiled a report to provide our stakeholders with information of some of the trends we have observed this year so far.
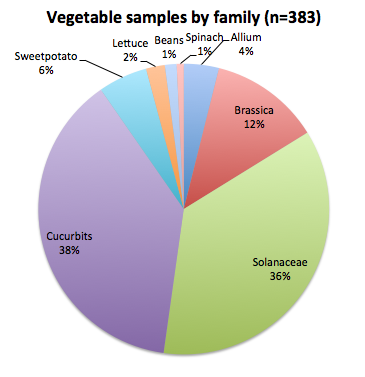
The clinic has received approximately 330 vegetable samples this year, and our lab has independently provided diagnostics for 53 additional samples as part of our collaborator role with the cucurbit downy mildew IPM pipe. The following figure shows the percentages of vegetables grouped by type/family, and indicates that the vast majority of samples we diagnose are cucurbit and solanaceous crops, followed by brassica crops, sweet potatoes, lettuce, beans and spinach.
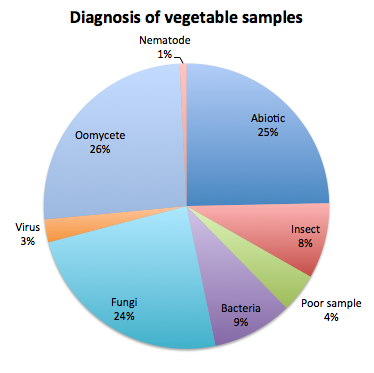
On the vegetable samples we cover at the clinic with the vegetable diagnostician Shawn Butler (all vegetables except for tomatoes and peppers) and our independently-diagnosed samples, we found that the most frequent diagnosis have been oomycete diseases (in order Downy mildew, Phytophthora, and Pythium), an abiotic cause (fertilization, soils, salts, water stress, injury, chemical burns, etc), and fungal diseases (in order Fusarium, gummy stem blight, and anthracnose). It is important to note that cucurbit downy mildew is highly represented in the oomycete disease group due to our work with this pathogen, but diagnostics of other downy mildews, Phytophthora and Pythium were still significant. We also identified some bacterial diseases such as bacterial leaf spots and fruit blotch, and a few virus and nematode-affected samples. Several samples submitted were affected by insect damage and no pathogens were found, and other samples could not be processed due to poor quality of the sample. The following figure summarizes this information:
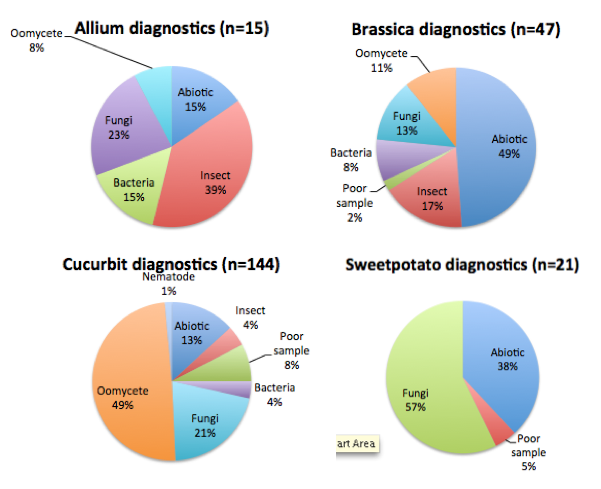
When we look more closely at the diagnostics by vegetable type of the crops we collaboratively diagnose with the clinic (all vegetables except tomatoes and peppers), we see differences in the most common diagnosis for these vegetables. The following figure shows the crops with more samples submitted (cucurbits, brassicas, sweet potatoes and alliums). For allium crops, the most common diagnosis was insect damage, followed by fungal diseases. For brassica crops abiotic causes were the most frequent diagnosis, followed by insect damage and fungal diseases. For sweetpotato more than half of the samples were affected by fungal diseases and others by abiotic causes. In cucurbits, oomycete diseases were the most common cause of crop damage, and if we remove the cucurbit downy mildew samples, fungi are the main cause of cucurbit disease.
Vegetable crops are most susceptible to disease when there is an underlying abiotic stress or injury, thus, having good soil, fertilizing and insect control strategies will results in a healthier crop.
Oomycete and fungal diseases that frequently affected crops this year include pathogens that are dispersed via air currents, and contaminated seed, soil, water or plant material.
For the airborne pathogens such as downy mildew, is important to use host resistance when available and a preventive spray program since there is little one can do to avoid exposing the crop to the pathogen.
For pathogens potentially being introduced into the field through contaminated seed or transplants such as gummy stem blight, is important to start with pathogen-free material, destroy any seedlings showing symptoms of disease and all neighboring seedlings, and protect the seed and transplants with fungicides if possible.
For pathogens potentially being introduced into fields through infested soil or irrigation water such as Phytophthora and Pythium, it’s important to take steps to determine if you have the pathogen present or not since they can survive in your soil for many years and have a broad host range, which limits the efficacy of crop rotation. If the pathogens are not present, do what you can to avoid introducing them by cleaning your equipment after visiting a field. If you have the pathogen in your field take steps to determine if your irrigation water may be contaminated, if it is, consider using deep well water for irrigation or incorporating a filtering system. If your water is clean, but the pathogen is in your soils, crop rotation to non-hosts will help, as well as cultural practices (use of plastic mulch and drip irrigation), and preventative spray programs. Some growers have used fumigation in cases where inoculum levels of soilborne pathogens are high.
We will continue to publish news and alerts about diseases affecting NC vegetable crops through the Extension Plant Pathology Portal.
Further information about disease control strategies for specific pathogens can be found in the North Carolina Agricultural Chemicals Manual.